
The AxiaLIF+® (Axial Lumbar Interbody Fusion) System is designed to create a safe and reproducible anterior retroperitoneal pre-sacral access route to the L5-S1 vertebral bodies.

TranS1’s foundational solution, AxiaLIF+, requires only a 2 cm incision size, which reduces blood loss, inpatient stay, and interoperative time – all while achieving a favorable fusion rate as high as 94% in peer-reviewed publications. [1, 5, 7, 8, 9]
Access, Discectomy, Grafting, Distraction, and Stabilization
The AxiaLIF+ instrumentation is designed to enable standard of care fusion principles, distraction, and stabilization of the anterior lumbar column, while mitigating the soft tissue trauma associated with traditional lumbar fusion.[1,2]
Every step of the AxiaLIF+® procedure – Access, Discectomy, Grafting, Distraction, and Stabilization – is designed to produce quality patient outcomes including excellent fusion rates and low complication rates with procedural efficiencies in mind.
Biomechanical Stability
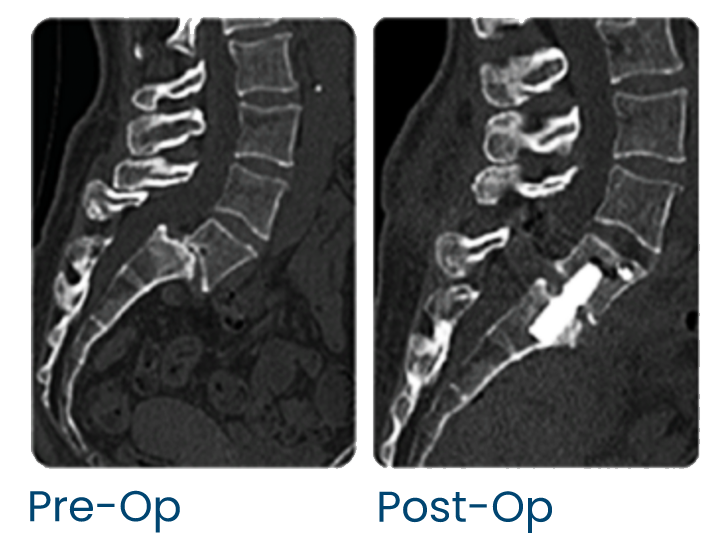
Numerous studies have documented the excellent fusion rate and biomechanical stability of AxiaLIF+.[8] The strength of the implant enables surgeons to build a strong foundation at the base of the spine.
Individualized Approach
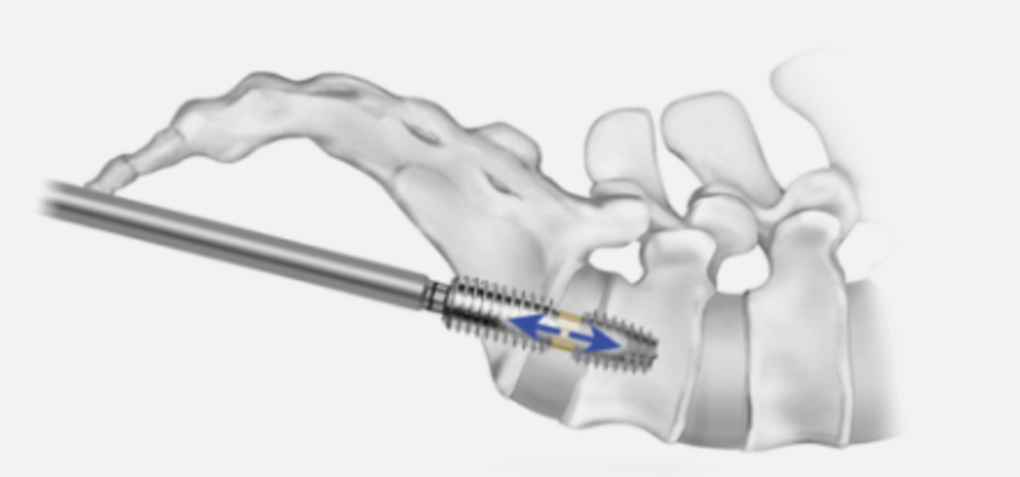
The internal distraction rod in AxiaLIF+ allows surgeons to individualize distraction in situ based on each patient’s needs. This highly adjustable distraction is achieved as the internal rod is advanced between robust, static anchors.

Shaped for Success
The bone-engagement surfaces on the superior aspect of the implants have a full taper design in which each revolution cuts new, larger threads in the endplate.

Minimal Trauma
The patented pre-sacral approach AxiaLIF+ causes less patient blood loss, less operating room time, and quick patient recovery.[1, 2, 3, 4, 10]
The AxiaLIF+ implant first received FDA 510(k)
clearance in 2006.
Arthrodesis, pre-sacral approach designated as a Category 1, non-experimental CPT Code (22586)
18,000+
procedures performed
Over 95 published research studies support the efficacy, safety and long-term outcomes of AxiaLIF+
- The AxiaLIF+ implant first received FDA 510(k) clearance in 2006.
- Arthrodesis, pre-sacral approach designated as a Category 1, non-experimental CPT Code (22586)
- 18,000+ procedures performed
- Over 95 published research studies support the efficacy, safety and long-term outcomes of AxiaLIF+
Training Opportunities
Make a Difference in Spine
Surgeon Resources

